PO4 3 Lewis Structure How to Draw the Dot Structure II lSCIENCE ll
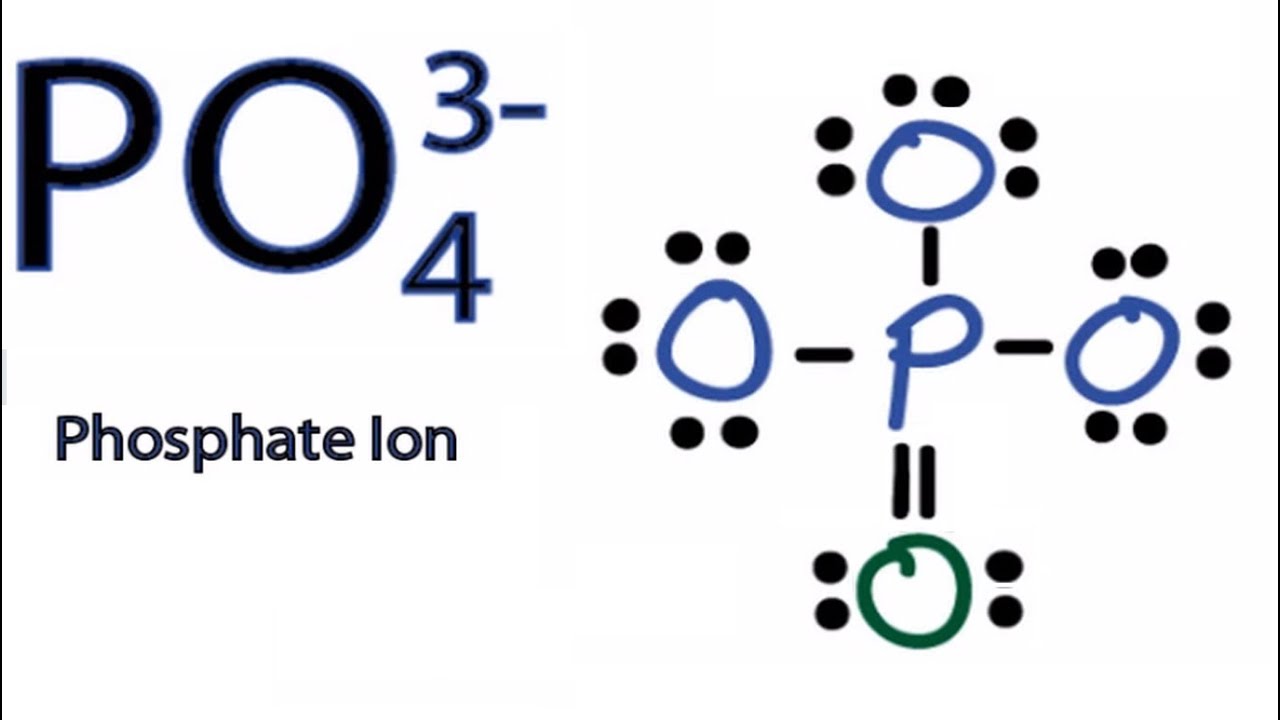
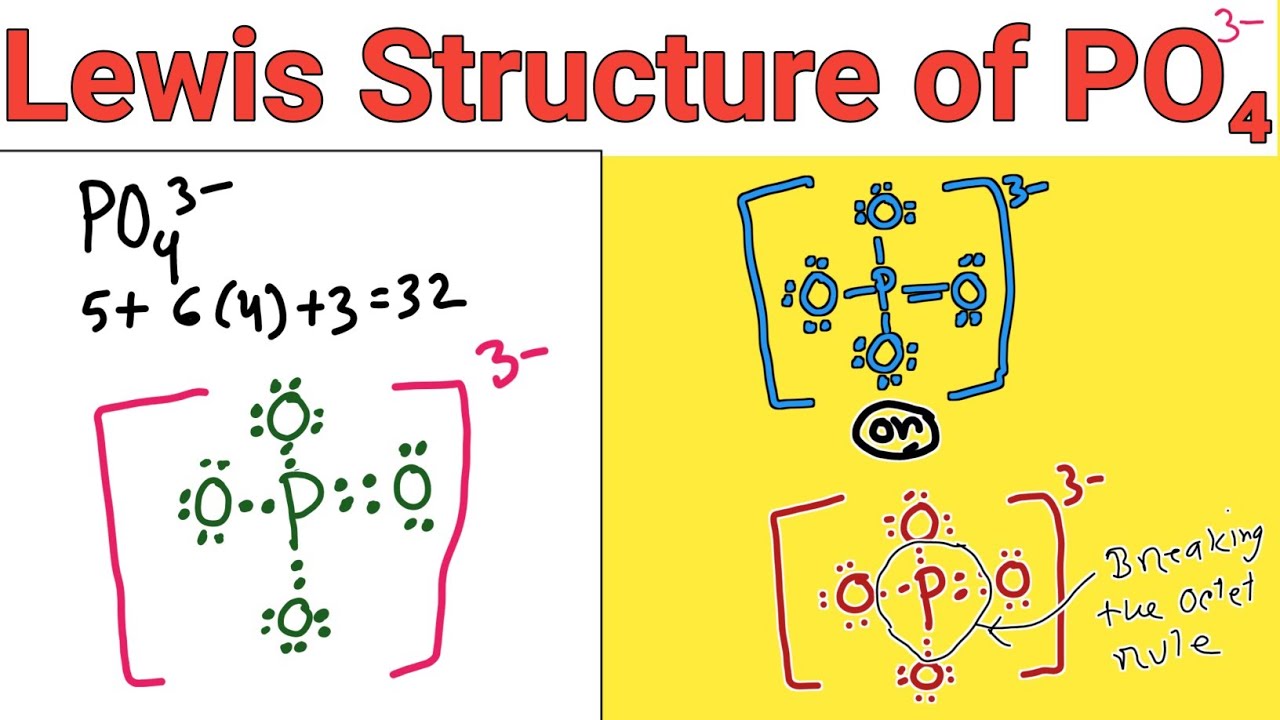
In the PO 43- Lewis structure, there is one double bond and three single bonds around the phosphorus atom, with four oxygen atoms attached to it. One oxygen atom with a double bond has two lone pairs, and three oxygen atoms with single bonds have three lone pairs. Also, there is a negative (-1) charge on the three oxygen atoms with single bonds.

Lewis Structure, Hybridization, Polarization, and Molecular Geometry of
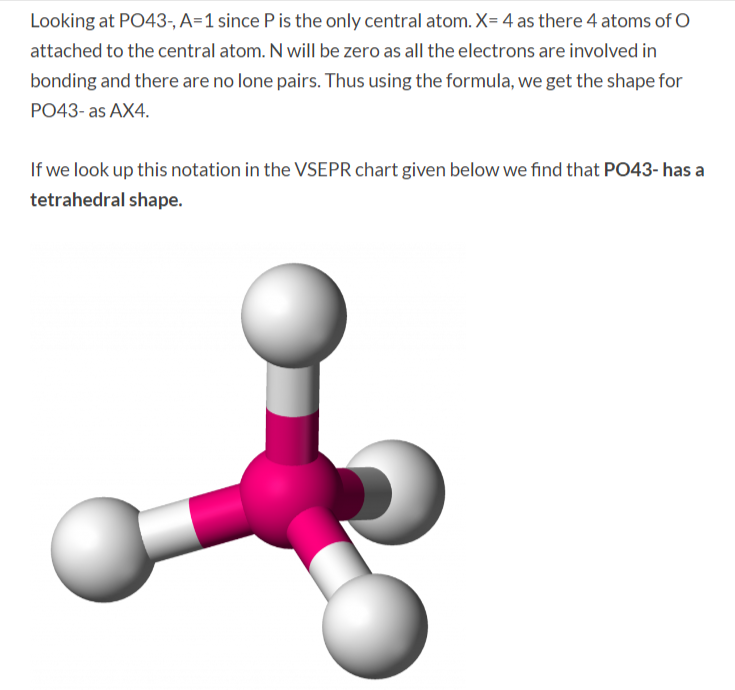
How to draw lewis structure of PO43-? The Lewis structure of a phosphate [PO4]3- ion consists of one phosphorus (P) atom and four atoms of oxygen (O). The phosphorus atom is present at the center while the oxygen atoms occupy terminal positions. There are a total of 4 electron density regions around the central phosphorus atom in [PO4]3-.
PO4 3 Lewis Structure How to Draw the Lewis Structure for PO43 YouTube
This chemistry video tutorial explains how to draw the lewis structure of PO4 3-, the phosphate ion. It also discusses the formal charge and resonance struc.

PPT Bonding General Concept PowerPoint Presentation, free download
Lewis structure of phosphate ion is drawn clearly in this tutorial step by step. Total valence electrons concept is used to draw the lewis structure of PO 43- ion. In lewis structure, there should be charges on atoms. Phosphate ion | PO 43- Phosphate ion is one of the oxyanion of phosphorous. Phosphorous is at +5 oxidation state in PO 43-.
SOLVED 23) Examine this Lewis structure for the phosphate ion, PO43
PO43- Molecular Geometry / Shape and Bond Angles Wayne Breslyn 726K subscribers Join Subscribe Subscribed 187 Save 56K views 10 years ago A quick explanation of the molecular geometry of PO43-.

What Is The Formal Charge On Phosphorus In A Lewis Structure Drawing Easy
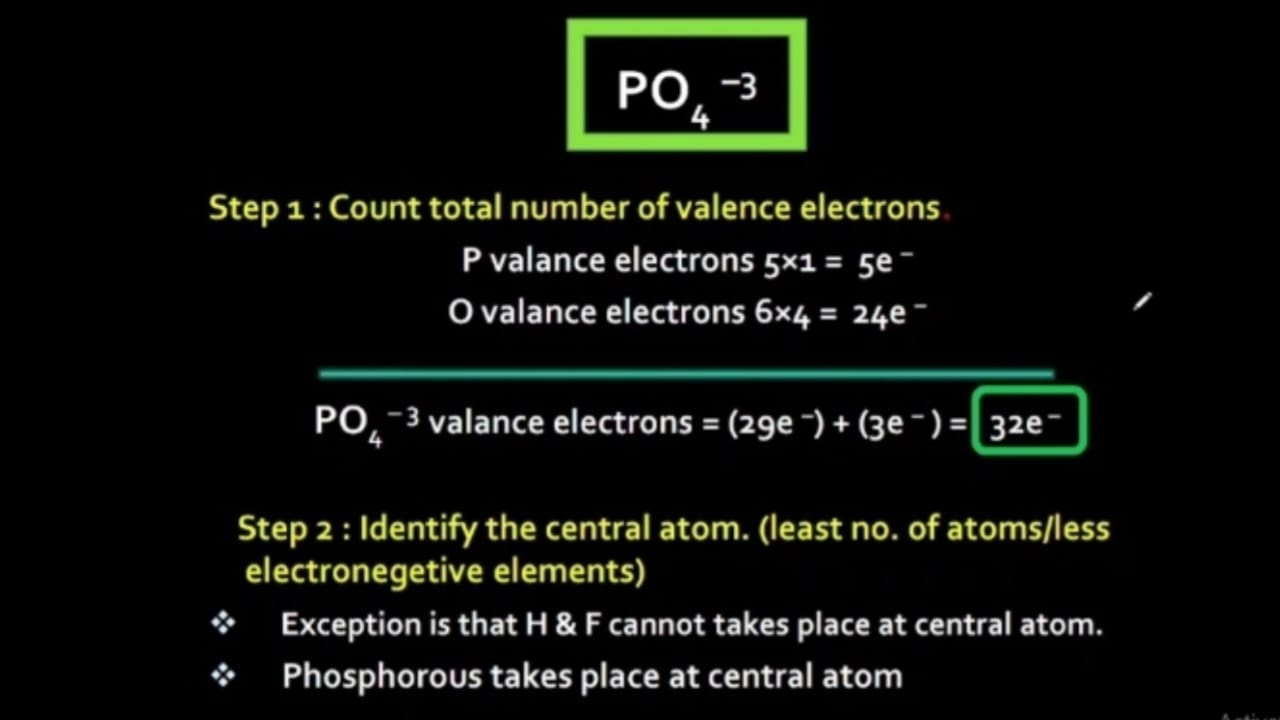
Steps of drawing PO4 3- lewis structure Step 1: Find the total valence electrons in PO4 3- ion. In order to find the total valence electrons in PO4 3- ion (phosphate ion), first of all you should know the valence electrons present in phosphorus atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
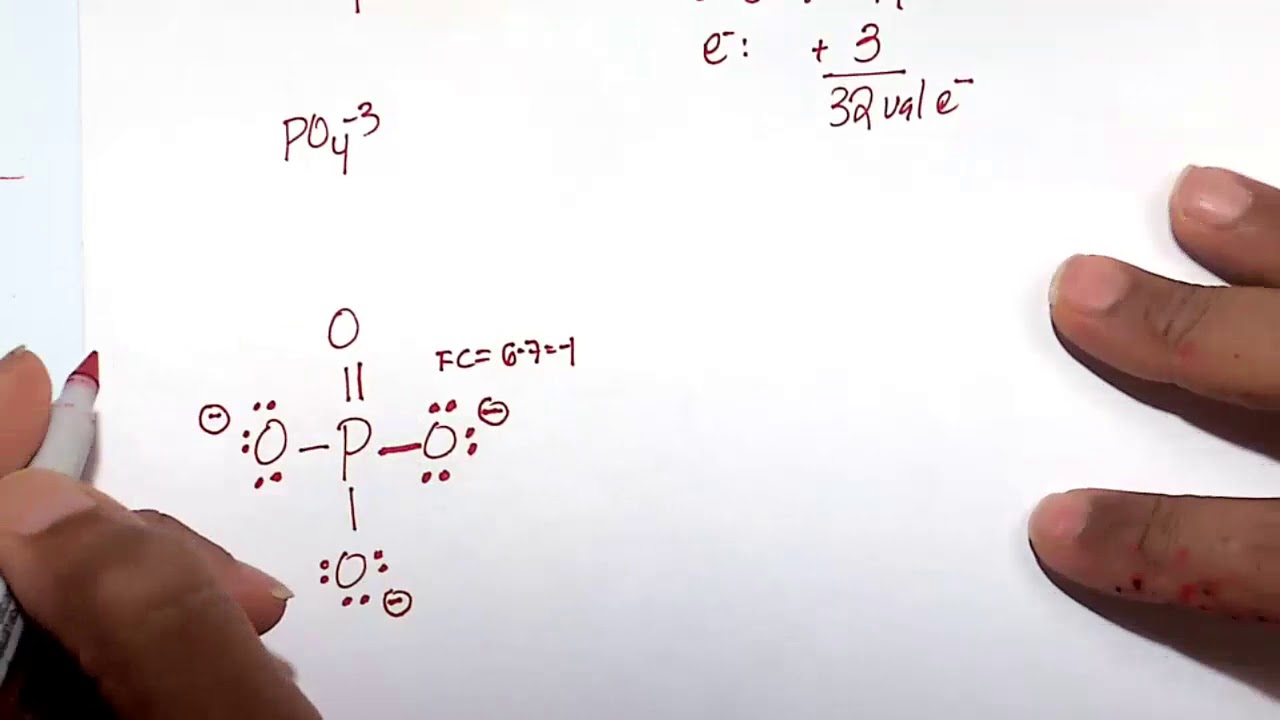
Resonance The resonance structure of the phosphate ion (PO4(3)) YouTube
PROBLEM 4.2.4 4.2. 4. Methanol, H 3 COH, is used as the fuel in some race cars. Ethanol, C 2 H 5 OH, is used extensively as motor fuel in Brazil. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas.

Resonance Structures for PO4 3 (Phosphate ion) YouTube
PO4 3- is a chemical formula for Floroform. It consists of one sulphur atom and four oxygen atoms.
Lewis structure of PO4 3 (Phosphate ion) YouTube
A step-by-step explanation of how to draw the PO43- Lewis Dot Structure (Phosphate ion).For the PO4 3- structure use the periodic table to find the total num.

PO43 lewis structure, molecular geometry, hybridization, and bond angle
In this video, Let us discuss how to write Lewis structure of PO43-, Phosphate ion easily. A simple notation used to represent valence electrons in an atom is called Lewis symbol. According.

Simple Method for writing Lewis Structures of the phosphate ion(PO4)3
Geometry. PO43- Geometry and Hybridization. Phosphorous is the central and there are 5 + 4×6 + 3 = 32 electrons. Give each oxygen three lone pairs: All the electrons are used and the only thing to fix is the ionic charge. As drawn, it would be a -4, therefore, we give one lone pair from an oxygen to make a double bond with the P which can.

Structure of PO43 ion Phosphate ion structure Lewis dot method
Lewis structure of PO43- ion (phosphate ion) contains one double bond and three single bonds between the Phosphorus (P) atom and Oxygen (O) atoms. The Phosphorus atom (P) is at the center and it is surrounded by 4 Oxygen atoms (O). Let's draw and understand this lewis dot structure step by step.

PO43 Molecular Geometry / Shape and Bond Angles YouTube
In the Lewis structure of PO43-, P forms single bonds with 3 oxygen atoms and forms a double bond with one oxygen atom. These oxygen atoms carry a charge of -1. Let us now look that steps required for drawing a Lewis structure:- 1. Counting the total number of valence electrons of the molecule. 2. Locating the central atom of the molecule. 3.
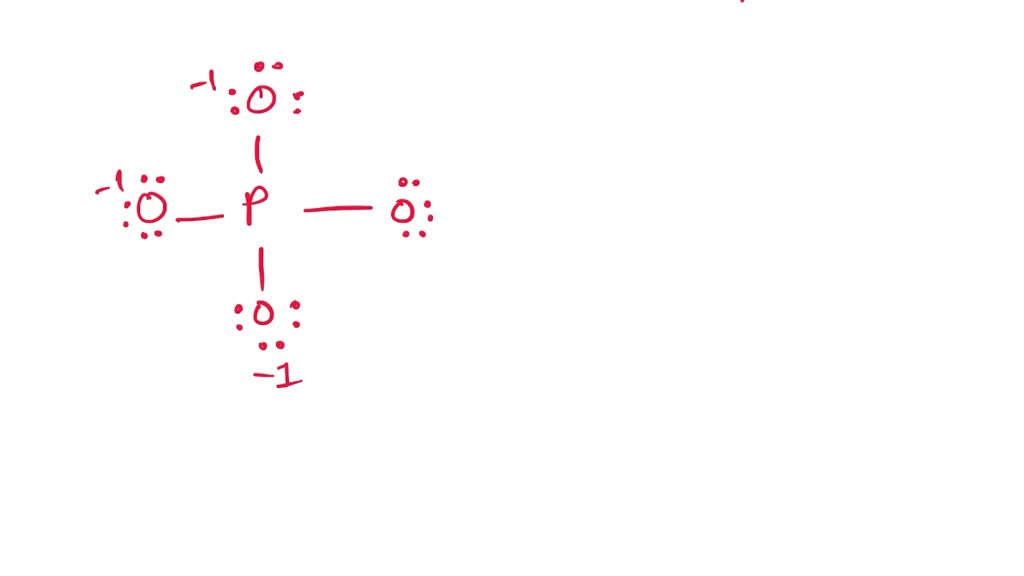
SOLVED The formal charge on the phosphorua atom in resonance structure
Hey Guys!Did you know that Phosphorus can have expanded orbitals and can accommodate more than 8 electrons in its outer shell? Well, such information helps t.

In PO43 , the formal charge on each oxygen atom and the P O bond order
In the Lewis structure of PO43- there are a total of 32 valence electrons. For the Lewis structure for PO4 3- you should take formal charges into account to find the best Lewis structure for the molecule. Remember, PO4 3- has a negative three charge on the molecule. For the Lewis structure you'll need to have a total charge for the molecule of 3-.
PO43LewisStructureHybridizationPolarityandMolecularGeometry
Phosphate is a very weak oxidizing agent. Since the phosphorus is in its highest oxidation state in phosphate ion, this ion cannot act as a reducing agent. This page titled Phosphate Ion (PO₄³⁻) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by James P. Birk. Phosphate ion is a reasonably strong base.